What is the formal charge of SO4?
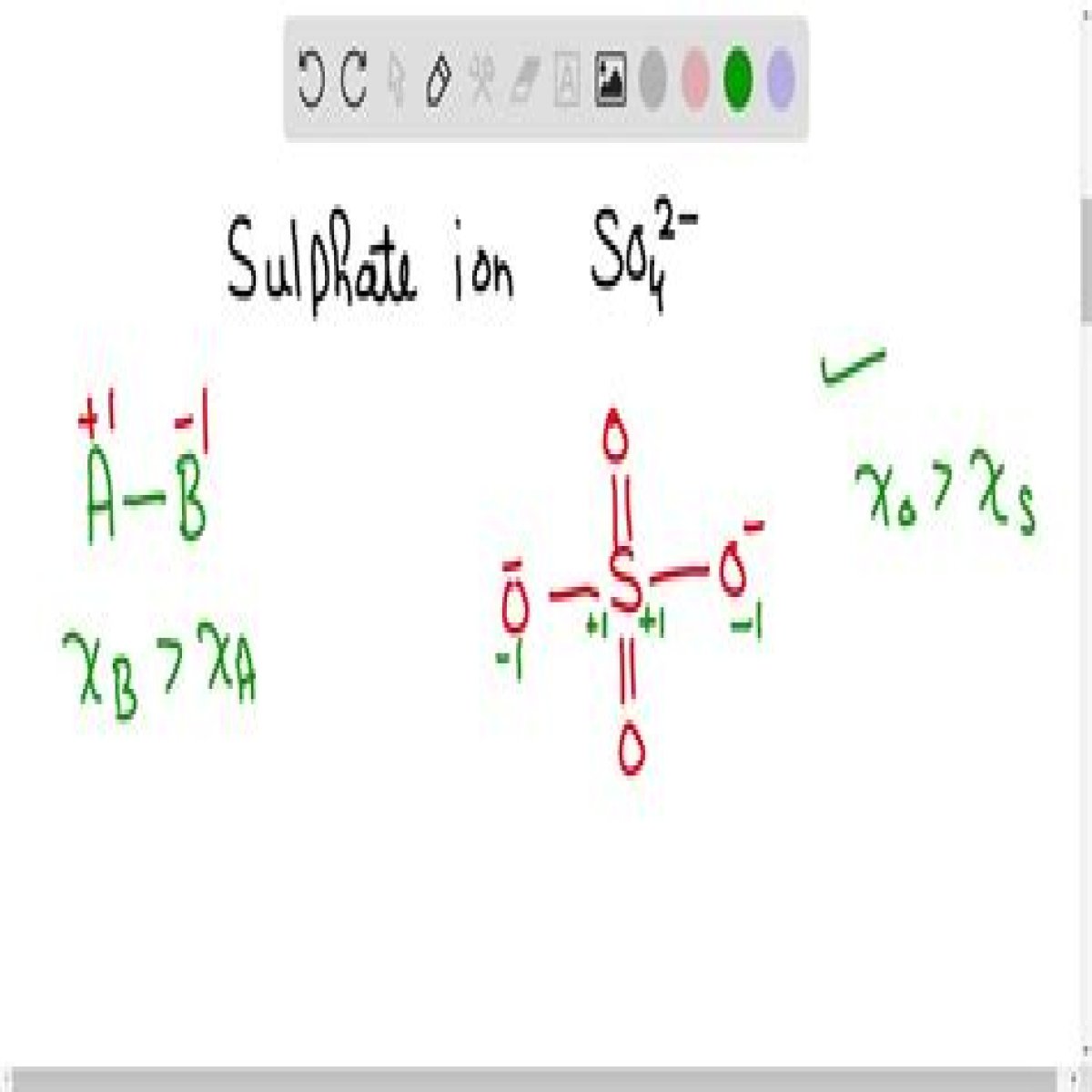
Charge on Sulfate Sulfate has a 2− charge. In order to understand why this polyatomic ion has a charge, the formal charge of one of the resonance structures can be observed. In the image below, the oxygen atom labeled 1 has 6 valence electrons. This is because it is in group VI on the periodic table.
What is sulfur’s charge in SO42 -?
The oxidation number of oxygen is almost always -2. For example, in sulfate ion (SO42-), each oxygen has an oxidation number of -2, whereas sulfur has an oxidation number of +6.
What is the formal charge on sulfur in SO42 where the Lewis structure of the ion is?
Because a free sulfur atom has six valence electrons, the sulfur is this diagram is assigned a formal charge of +2.
What are the formal charges on S and O in SO42 If S obeys the octet rule?
The formal charge of S in SO4-2 ion that obeys the octet rule is +2.
How is SO42 formed?
The sulphate ion is mainly composed of sulphur and oxygen atoms. Here, sulphur is the central atom and it is surrounded by four oxygen atoms that are located at equal distances in the plane. As for the bonding, 2 of the oxygen atoms form S=O. bonds and the other two form S-O- bonds.
What is the formal charge of alcl4?
-13.1Computed Properties
| Property Name | Property Value | Reference |
|---|---|---|
| Formal Charge | -1 | Computed by PubChem |
| Complexity | 19.1 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Isotope Atom Count | 0 | Computed by PubChem |
| Defined Atom Stereocenter Count | 0 | Computed by PubChem |
How do you calculate formal charge and resonance?
Calculating Formal Charge Another way of saying this is that formal charge results when we take the number of valence electrons of a neutral atom, subtract the nonbonding electrons, and then subtract the number of bonds connected to that atom in the Lewis structure.